Authors:
Hojat Asgariandehkordi、Sobhan Goudarzi、Mostafa Sharifzadeh、Adrian Basarab、Hassan Rivaz
Paper:
https://arxiv.org/abs/2408.10987
Introduction
Ultrasound imaging is a cornerstone in modern medical diagnostics due to its non-invasive nature and cost-effectiveness. It provides real-time visualization of internal structures, aiding in the detection and diagnosis of various medical conditions. However, the presence of noise in ultrasound images can significantly impair their interpretability and diagnostic accuracy. This noise can stem from various sources, including electronic noise and acoustic artifacts, which collectively degrade image quality.
To address these challenges, robust post-processing techniques have been developed to enhance ultrasound image quality. Traditional methods such as image filtering, speckle reduction algorithms, and contrast enhancement have been employed, but they often struggle with the complexities inherent to ultrasound data. Recently, deep learning has emerged as a powerful tool in medical imaging, offering significant improvements in noise reduction and image enhancement.
In this study, we explore the use of Denoising Diffusion Probabilistic Models (DDPMs) for denoising plane wave (PW) ultrasound images. DDPMs, a type of generative model, have shown remarkable performance in image denoising tasks. Our approach leverages the iterative denoising capabilities of DDPMs to improve the quality of ultrasound images, focusing on enhancing Radio Frequency (RF) beamformed data while preserving essential image features.
Related Work
Ultrasound Image Denoising Methods
Ultrasound image quality can be degraded by various factors, including Gaussian electronic noise and acoustic noise sources such as reverberation and multiple scattering. Traditional denoising methods include adaptive filtering, wavelet-based denoising, anisotropic diffusion filters, and non-local means denoising. While these methods have been effective to some extent, they often suffer from high computational complexity and can produce blurry outputs.
Deep learning-based methods have shown promise in improving ultrasound image quality. Techniques such as U-Net, Mimicknet, and self-supervised learning have been employed for tasks like image denoising and reconstruction. These methods leverage large-scale datasets and intricate patterns within ultrasound data to enhance image quality.
Generative Models
Generative models, including Generative Adversarial Networks (GANs), Variational Autoencoders (VAEs), and diffusion models, have been explored for image denoising and enhancement. GANs excel in high-quality sample generation but face challenges in mode coverage diversity. VAEs offer fast inference and mode coverage diversity but may not generate high-quality samples. Diffusion models, on the other hand, are known for their high-quality sample generation and mode coverage diversity, albeit with greater computational complexity.
DDPMs, a type of diffusion model, have shown strong performance in denoising tasks by progressively reducing noise through iterative steps. This study leverages DDPMs to enhance the quality of PW ultrasound images, focusing on transitioning from low-quality to high-quality images.
Research Methodology
Forward and Reverse Processes
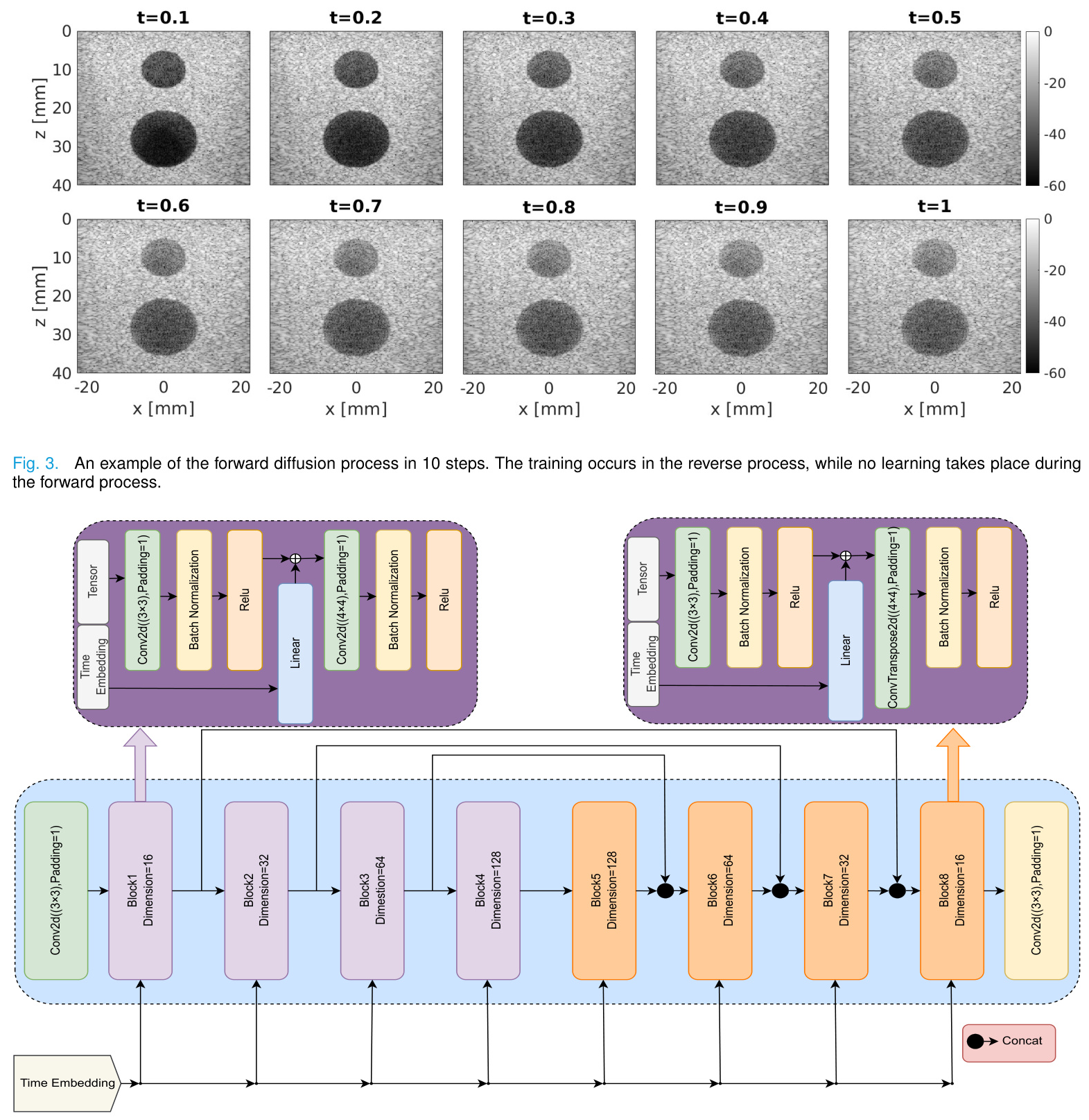
DDPMs consist of two main processes: the forward process and the reverse process. In the forward process, an image is progressively perturbed by adding noise over several steps. The reverse process involves starting from a noisy image and iteratively enhancing its quality to approximate the original high-quality image.
-
Forward Process: Given an initial image ( x_0 ), the forward process perturbs the image by adding noise at each step:
[
x_t = \sqrt{1 – \beta_t} x_{t-1} + \sqrt{\beta_t} \epsilon, \quad \epsilon \sim \mathcal{N}(0, I)
]
where ( x_t ) is the data at time step ( t ), ( \beta_t ) is a variance schedule, and ( \epsilon ) is noise sampled from a normal distribution. -
Reverse Process: The reverse process aims to transition from a noisy image at step ( T ) to a high-quality image ( x_0 ):
[
P_{\theta}(x_0) = \int p_{\theta}(x_{0:T}) \, dx_{0:T}
]
The objective is to maximize the likelihood of the predicted image ( P_{\theta} ), where ( \theta ) denotes the prediction parameters.
Proposed Method
Our method adapts the forward and reverse processes to directly transition between low-quality and high-quality ultrasound images without relying on normal noise distributions. The forward process interpolates between low-quality and high-quality images, while the reverse process enhances the image quality step by step.
-
Forward Process:
[
x_t = (1 – t) X_0 + t X_1
]
where ( X_0 ) represents high-quality images and ( X_1 ) represents low-quality images. -
Reverse Process:
[
x_{t – \Delta t} = x_t – v_{\theta}(x_{t – \Delta t}, t) \Delta t
]
The reverse process is deterministic, ensuring consistent output for a given input.
Architecture
The proposed architecture is inspired by U-Net and consists of convolutional blocks and time embedding modules. The time embedding module provides temporal context to the network, while the convolutional blocks process the input tensor and time vectors to enhance image quality.
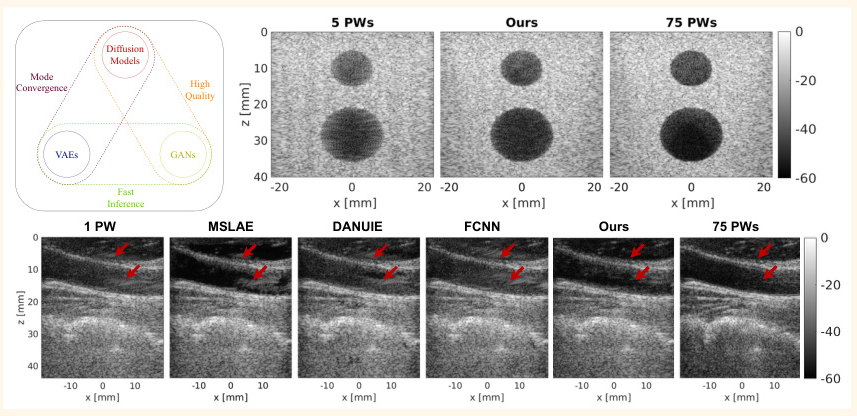
Experimental Design
Dataset
The proposed method was trained on a simulated dataset and evaluated on both phantom and in vivo data.
-
Simulated Data: The simulated dataset consists of 400 images generated using the Field II package. The images contain fully developed speckle patterns and various echogenicity types. The dataset was divided into training, validation, and test sets.
-
Phantom and In Vivo Data: The method was evaluated on experimental phantom and in vivo images from the PICMUS benchmark dataset. The phantom images represent areas with anechoic cysts, while the in vivo images depict a volunteer’s carotid artery.
Evaluation Metrics
The performance of the proposed method was evaluated using the following metrics:
-
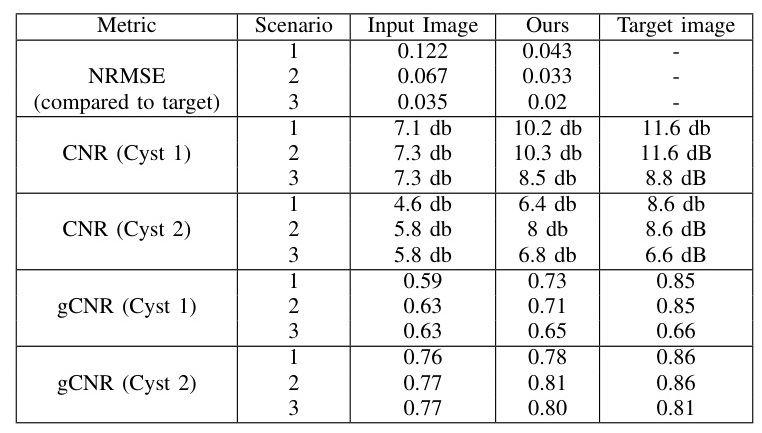
Contrast to Noise Ratio (CNR):
[
\mathrm{CNR} = 20 \log_{10} \left( \frac{|\mu_{\mathrm{ROI}} – \mu_B|}{\sqrt{(\sigma_{\mathrm{ROI}}^2 + \sigma_B^2) / 2}} \right)
] -
Generalized Contrast to Noise Ratio (gCNR):
[
\mathrm{gCNR} = 1 – \int_{-\infty}^{\infty} \min { p_{\mathrm{ROI}}(x), p_B(x) } \, dx
] -
Normalized Root Mean Squared Error (NRMSE):
[
\mathrm{NRMSE} = \frac{\mathrm{RMSE}}{\max(Y)}
] -
Kolmogorov-Smirnov (KS) Test: Quantifies the differences between the cumulative distribution functions (CDFs) of pixel intensity values in the images.
-
Structural Similarity Index (SSIM): Measures the similarity between two images, considering luminance, contrast, and structure.
Training
The network was trained using an Adam optimizer with a learning rate of 0.004, adjusted by a linear scheduler. The training procedure encompassed 350 epochs, and the network was trained on simulated images of size 1082×192.
Results and Analysis
Simulated Test Data
The proposed method was evaluated on simulated test images across three scenarios with different low-quality and high-quality pairs. The results demonstrate significant improvements in image quality, as evidenced by the quantitative metrics.
Phantom Data
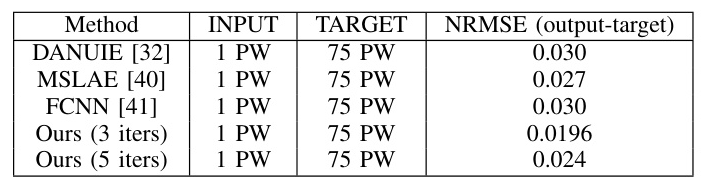
The method was compared with other denoising techniques on phantom data. The proposed method outperformed other methods in terms of CNR, gCNR, and SSIM, demonstrating superior denoising capabilities.

In Vivo Data
The method was further evaluated on in vivo data, showing enhanced visibility of the artery and reduced clutter artifacts. The proposed method achieved the closest approximation to the target image, outperforming other methods.
Overall Conclusion
This study presents a novel approach for denoising plane wave ultrasound images using Denoising Diffusion Probabilistic Models (DDPMs). The proposed method effectively enhances the quality of RF beamformed data while preserving essential image features. Trained on a small dataset of simulated images, the method demonstrated strong performance on both phantom and in vivo data.
The iterative denoising capabilities of DDPMs, combined with the proposed forward and reverse processes, enable significant improvements in image quality. The method outperforms traditional and deep learning-based denoising techniques, offering a promising solution for enhancing ultrasound images in clinical applications.
Future work will focus on further validation with larger datasets, exploring the impact of denoised RF data on downstream tasks, and optimizing the processing time for real-time performance. The adaptability of the proposed method to different ultrasound modalities and transducer configurations underscores its potential for broad applicability in medical imaging.